Building a microscope for simultaneous imaging and precise optogenetic activation
 Light-sheet microscope with SLM integration.
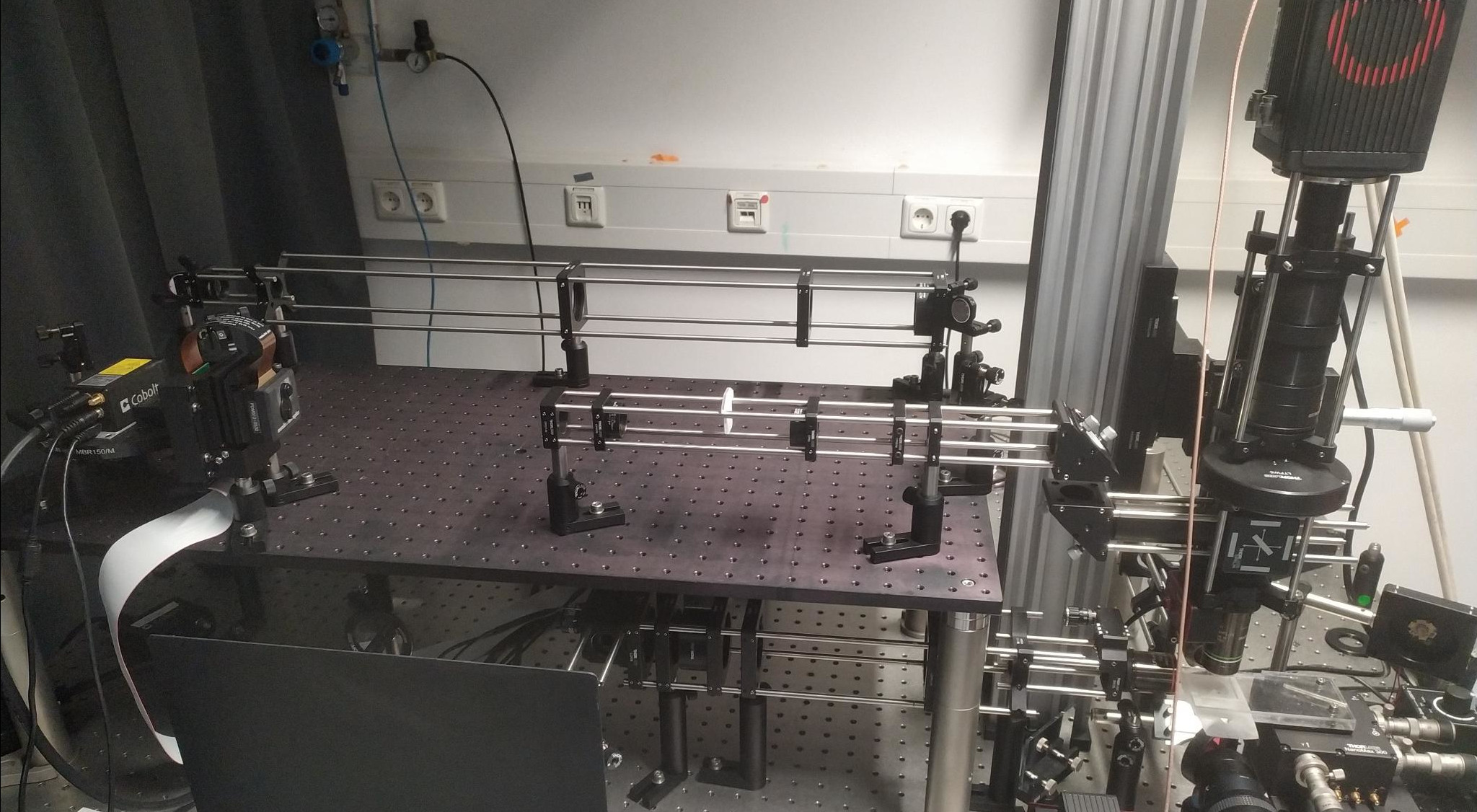
Light-sheet microscope with SLM integration.
The Quick Take
In neuroscience, we often aim to understand the brain by presenting a subject with certain stimuli while simultaneously recording their behavioural and neural responses. These recordings then give us insights into how the brain works. A powerful way to probe brain function is by actively manipulating neural activity - exciting or inhibiting specific brain regions - and observing the resulting changes. The goal of this project was to integrate a Spatial Light Modulator (SLM) into a light-sheet microscope to precisely target light and manipulate specific brain regions during experiments, potentially enabling new insights. Although I was able to add the SLM to the microscope and target specific areas of the brain, it was not possible to reach the desired precision within the timeframe of the project.
Introduction
In the Portugues Lab, most experiments involved presenting zebrafish with some stimuli, and recording both their brain activity and behaviour. This was done by shining blue light on them, which caused their neurons to fluoresce due to the expression of GCaMP, a calcium-sensitive fluorescent protein. Because zebrafish are transparent, microscopes like two-photon (2P) or light-sheet systems can image their brain activity directly, without the need for invasive measures.
Just like taking apart an old device reveals how its components interact, understanding the brain requires not just observation but also intervention. By the same principle, every neuroscientist and psychologist knows about H.M. and Phineas Gage, because the disruption of brain function revealed so much about the inner workings of the brain. This is where optogenetics - a technique that allows researchers to excite or inhibit specific neurons using light - comes into play.
Generally, optogenetic experiments are carried out by flashing light on the whole brain. However, this does not allow for precise spatial control, especially because restricting or changing the expression of the optogenetic gene to a specific brain region is tedious. It also makes it difficult to dynamically change which neurons are targeted over time.
That's why in this project, the goal was to gain more precision with the light used to control the neuronal activity. Specifically, I wanted to integrate a Spatial Light Modulator (SLM) into a light-sheet microscope. Whereas the light-sheet microscope creates a thin-sheet of light and moves it over the brain to pick up on the GCaMP fluorescence, a Spatial Light Modulator reshapes the incoming laser light by adjusting its wavefront. By carefully controlling the phase of the light, we can create illumination patterns at the target - very similar to Fraunhofer diffraction. These patterns are designed to align with the locations of the neurons of interest.
Execution
I first tested the SLM outside the microscope to see how it interacted with the other components, to develop and test the pattern-generation algorithms, and to ensure that the SLM could be controlled effectively.
Then, the part of the microscope that involved the SLM had to be designed. At a high level, the collection path - originally designed to transmit only green fluorescence - had to be modified to pass the optogenetic stimulation light to the sample plane, but prevent it from going to the sensor. Furthermore, the laser beam used for stimulation had to be expanded to fully illuminate the SLM, and then refocused again to match the size of the sample plane. The final optical setup is shown above.
Once the optical setup was complete, I tested whether the integration of the SLM was successful. This was done by taking two groups of fish with the same background: one group expressed only the gene for imaging brain activity, while the other also expressed the gene required for optogenetic control. If the targeted region showed a difference in activity for the optogenetic fish but not for the control group, it would indicate that the SLM integration was successful.
Results
Initial tests targeting large brain regions showed clear modulation of neural activity, confirming that the SLM was integrated successfully. After confirming the basic functionality, I started testing the SLM's spatial precision by targeting smaller regions. However, these tests did not show the expected modulation. Further testing suggested that misalignment in the microscope was the likely cause. This is a natural process that happens over time due to (small) disturbances and disrupts the stimulation light. Unfortunately, it was not possible to solve this issue within the timeframe of this project. I concluded that while the system was able to target large regions, its exact spatial resolution could not be determined within the project timeline.
 Nathan
Nathan